NOTES PREPARED BY
ASHAQ HUSSAIN BHAT
TEACHER SCHOOL
EDUCATION DEPARTMENT
JAMMU AND KASHMIR
CARBON AND IT'S COMPOUNDS
Q.1 How does carbon occur in nature?
Ans. Carbon is one of the most widely distributed element, found in earth's crust. It occurs both,
In free state, carbon occurs as diamond, graphite and coal. Diamond and graphite are pure forms of carbon. Coal is an impure form of carbon in which percentage of carbon varies. In combined state carbon occurs as-
(i)Oxides such as carbon dioxide, and carbon monoxide.
(ii)In the form of natural gas, petroleum products, marsh gas.
(iii)Carbonates in the form of metal carbonates such as. Magnesium carbonate, (MgCO₂), Calcium carbonate, (CaCO)), Zinc carbonate, (Zn CO₂).
(iv)In the form of bio-molecules like proteins, fats, carbohydrates etc.
Q. Describe the position of carbon in periodic table.
Carbon is a non-metallic element having symbol "C'. It has an atomic number of 6 and mass number of 12u. It occurs in three isotopic forms such as 6C12, "6C13 and 6C14 Its electronic configuration is K2. L4
Where K&L are 1st and 2nd shells of carbon respectively.
It belongs to group 14 and is the first member of the group. The other members of the group are Si, Ge, Sn and Pb. Since it occurs at the top of the group 14, this group is also known as carbon family.
Q .Discuss unique nature of carbon.
Ans:- The unique nature of carbon can be explained in the following points.:-
i)Carbon has the self linking property to form chains of varying lengths and shapes (straight. branched, closed). This property of carbon is known as catenation.
ii)Carbon differs from the next of the elements of its group because of its smaller size and higher electro-negativity.
iii)Carbon shows unique ability to form multiple bonds such as C=C, C≡C, C=0, C ≡ N etc.
Iv)Carbon can bind to almost every elements in periodic table especially O, H, S etc.
V)Carbon forms complex molecules, therefore the no. of isomers increases.
Q . State the reason, why carbon always forms covalent bonds?
Ans. Carbon has six electrons and its electronic configuration is: K 2 L4
Thus, an atom of carbon contains four electrons in its outer shell (L-Shell). From the electronic configuration of carbon, it seems that carbon can form compounds in three different ways:
1 .By losing all four outer most electrons to form C4+ ion.
2 .By gaining four electrons to form C4-ion.
3 .By sharing all the four outer most electrons present in L-Shell and forming four covalent bonds.
Since carbon atom is small in size, so its outer most electrons are strongly bound to the nucleus and therefore large amount of energy is needed to remove electrons from a carbon atom.
As a result carbon atom shows no tendency to lose its valence electrons. On the other hand, due to low nuclear charge, carbon is moderately electronegative, so it does not show any tendency to gain electrons. Therefore, the option left for carbon to form compounds is only by sharing its four electrons forming four covalent bonds to complete its octet.----
Q. What is allotropy? Give the allotropic forms of carbon.
Ans:-The phenomenon of existence of an element in two or more different forms having different physical properties but similar chemical properties is known as allotropy and the various forms as allotropes or allotropic forms. Carbon, sulphur and phosphorus are some of the non-metals that show allotropy. Carbon exists in two allotropic forms:
(1) Crystalline
(2)Amarphus
1 . Crystalline forms of carbon:- crystalline forms of carbon are:
a) Graphite
b) Diamond
c) Fullerenes
2 . Amorphous forms of carbon:- The various amorphous forms of carbon are coal, coke, charcoal, bone or animal charcoal, carbon black etc. Among all the known forms of carbon, diamond, graphite and fullerenes are the purest forms.
Q. Give a detailed account of Diamond. (occurrence, structure, properties and uses)
Ans:- Diamond is the purest form of carbon. The word diamond comes from the two Greek words diaphones means transparent and adamas means extremely hard. Diamond is found in all shapes and sizes. Diamond is found in ancient valcano pipes where it is generally embedded in asoft dark coloured rock called blue ground or Kimberlite rocks.
Diamond can also be prepared artificially by subjecting carbon to very high pressure and temperature. These synthetic diamonds are small but are otherwise indistinguishable fom natral diamonds.
Occurrence:
Diamond deposits have been found in South Africa, Ghana, Angola, India, Brazil and Eastern Siberia.
In India, diamond are found in Panna (Madhya Pradesh), Wajrakarur (Andrapradesh) and Golconda (Karnataka). The famous Kohinoor diamond was found is Wajrakarar.
Structure:-
In diamond each carbon atom is linked to four other carbon atoms directed towards the corners of a regular tetrahedron through covalent bonds. The arrangement gives rise to a closely packed, hard, three dimensional structure which makes the diamond hardest natural substance. All the four valence electrons are engaged in forming carbon- carbon bonds, leaving no free electron. This makes diamond poor conductor of electricity.
Properties of Diamond:-
1.Diamond is a transparent solid having extra ordinary brilliance.
2.It is usually colourless, but we can impart colour to diamond by adding small amount of impurities in the form of metal salts.
3.It is a poor conductor of electricity, but good conductor of heat.
4.It has a high density of 3.5g/cm³.
5.It has a high refractive index of 2.5.
6.It is the hardest natural substance. One can cut a diamond with only diamond.
Use of Diamond:-
1. It is used in Jewellery because the property cut and polished diamond sparkles brightly.
2.It is used to cut glasses.
3. It is used for cutting and drilling of rocks.
4. It is used to make radiation proof windows in space satellites because it has ability to keep out harmful radiations.
5. Due to its extra-ordinary sensitivity to heat rays diamonds are used for making high precision thermometers.
Q. Give occurrence structure properties and uses of graphite.
Ans. Graphite is a crystalline form of carbon. It finds its name from the Greek word "grapheine" means to write. It is also called black lead because it marks paper black like lead. The chemical symbol of graphite is C.
Occurrence:- Graphite occurs free in nature and is widely distributed throughout the world. Major producers of graphite are USSR, Mexico, India, China, Canada and Srilanka. In India graphite is found in Orissa, Rajasthan, J&K State, Bihar, Karnataka, Tamilnadu etc. Graphite can also be prepared artificially by heating coke to a high temperature.
Structure of Graphite:- The structure of Graphite is altogether different from that of diamond. A graphite crystal actually consists of sheets or layers of Carbon atoms. In a graphite layer, each carbon atom is bonded to three other carbon atoms in the same plane forming hexagonal rings. To satisfy the fourth valency of carbon, each hexagonal ring has three alternate single and double bonds. The various layers are held together by weak Vander - walls forces of attraction. The distance between any two successive layers is 340 pm.
Properties:-
1. Graphite is an opaque, grayish-black in colour, with hexagonal crystals.
2. It is soft and greasy to touch.
3. It is a good conductor of heat and electricity.
4. Its density is 2.2 g/cm³.
5. It is stable to heat and possesses a high melting point of around 3700°C.
6. It has a metallic lustre.
Uses:-
1. It is used as a lubricant in fast moving machinery as graphite is soft and -slippery.
2. It is used to make electrodes in batteries and electric furnaces.
3. It is used to make the core of lead pencils as it is soft and can mark paper.
4. It is used to make black paints and in printer inks.
Q. Comparison of properties of Diamond and Graphite.
Ans:-The difference between the properties of diamond and graphite is summarized as below:-
Diamond. Graphite.
1. Diamond is the hardest substance. 1. Graphite is soft and soapy to touch known.
2. Diamond has a density of 3.5 g/m². 2. Graphite has a density of 2.3 g/m³.
3. Diamond is transparent and has a high. 3. Graphite is black and is opaque.
refractive index.
4. Diamond is a non-conductor of heat. 4. Graphite is a good conductor of
and electricity. and electricity,
5. Diamond. occurs as octahedral. 5. Graphite occurs as hexagonal rings.
Crystal
Q. Write a short note on Fullerenes.
Fullerenes are a class of carbon allotropes. They are spherical in shape and contain even no. of carbon atoms ranging from 60 to 350. The C60 fullerene is the most stable and was first to be identified. It contains 60 carbon atoms which are arranged in the shape of a foot ball, therefore it is also called as bucky ball.domes
These allotropes look like geodesic domes designed by the US Architect Buckminster Fuller, they are called as Buckminster fullerenes. Buckminster fullerene is dark solid at room temp. The properties of fullerene lie between diamond and graphite.
Compounds of Carbon.
The compounds of carbon can be classified into two categories.
1. Inorganic compounds
2. Organic Compounds
1. Inorganic Compounds:- These are the compounds of carbon with metals and non-metals (other than hydrogen). These do not have carbon- carbon bonds in them.. these compounds are generally obtained from mineral sources. e.g. Salt from Sea. metal oxides from soils etc.
2.Organic compounds:- These are the compounds of carbon and hydrogen and their derivatives. These contain carbon - carbon bonds. Organic compounds are mostly derived from living organisms e.g. sugar from sugarcane, oils from vegetables, proteins from eggs etc.
Q.10. What are Hydrocarbons? Give the types of hydrocarbons.
Ans. The compounds containing only carbon and Hydrogen are called hydrocarbons i.e.
Carbon+Hydrogen ⟶ Hydrocarbon
e.g. methane (CH4), ethane (C₂H6), Ethene (C₂H4), . The natural source of hydrocarbons is petroleum and natural gas. Both petroleum and natural gas occurs deep inside the earth. Hydrocarbons are regarded as parent organic compounds and all other organic compounds are considered the derivatives of hydrocarbons.
There are two main types of hydrocarbons.
1. Saturated Hydrocarbons.
2. Unsaturated Hydrocarbons.
1. The hydrocarbons in which all carbon atoms are bonded to each other by single covalent bonds are called as saturated hydrocarbons. Saturated hydrocarbons are also called as alkanes or paraffins. The general formula of alkanes is
CnH2n+ 2 where n= 1,2,3.........
e.g. If n = 1, the alkane is C₁H₂ (1) + 2 CH4 (methane)
if n = 2, C₂H2x2+2 = C2 H6 (ethane)
Ifn=3, C3H₂x 3 +2 = C3 H8 (Propane)
Ifn=4, C4 H10 (Butane)
Ifn=5, C5H12 (Pentane)
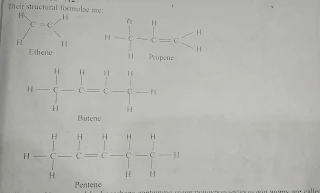
Their structural formulae are as follows
Any successive members of alkane differ by CH₂ unit. CH₂ unit is called methylene group.
Unsaturated Hydrocarbons:-
The hydrocarbons which contains double (=) or triple (≡) bonds between carbon atoms are called unsaturated hydrocarbons. Unsaturated hydrocarbons are of two types.
(i) Alkenes:- Unsaturated hydrocarbons containing double bond between carbon atoms are called as alkenes. Alkenes are also called as olefins. The general formula of alkenes is C₁ H₂n where n = 2,3, 4....... e.g.
Ifn=2, C2 H2x2. C₂H4. Ethene
Ifn=3, C3 H2x3. = C3 H 6. Propene
Ifn=4, C4 H2x4. = C4 Hs. Butene
Their structural formulae are as;
(ii) Alkynes:
Unsaturated hydrocarbons containing triple bonds between carbon atoms are called
as alkynes. The general formula of alkynes is CnH2n - 2. Where n = 2, 3, 4, 5.......
Ifn=2, C₂H2x2-2 = C₂H₂ ethyne
If n = 3, C3H2x3-2- C3H4 propyne
If n = 4, C4H2x4-2= C4H6 butyne
If = 5, C₂H2x5-2= C5H8. Pentyne
There structure formulae are as follows:
Cyclic Hydrocarbons:-
The hydrocarbons in which carbon atoms are arranged in a ring are called as cyclic
hydrocarbons. The cyclic hydrocarbons can be saturated or unsaturated.
a) Saturated cyclic hydrocarbons :- Cyclohexane with molecular formula C6 H₁2 is a saturated cyclic hydrocarbon.
Q) What is covalent bond? Give different types of covalent bond?
Ans;- Covalent bond:- The chemical bond formed by mutual sharing of electrons between two atoms in order to acquire stable nearest noble gas electronic configuration is called covalent bond. The two combining atoms may be similar or dissimilar atoms. The difference between the electro negativities of the combining atoms should be either zero or very small. The compounds which contain covalent bonds are called covalent compounds. The covalent bond is generally formed between two non-metallic elements. The shared pair of electrons becomes the property of both the bonded atoms. For example,
H• + •H ➡H•• H. Or H-H
Depending upon the number of electron pairs shared between the two bonded atoms, covalent bond is of three types:-
1. Single covalent bond
2. Double covalent bond
3. Triple covalent bond.
1. Single Covalent Bond:- Single covalent bond is formed by sharing of one electron pair between the two atoms. Example.
Formation of Hydrogen. (H₂) molecule:
Hydrogen atom has one electron in its shell. In order to attain electronic configuration of He, it shares its electron with another hydrogen atom. Thus there is a single covalent bond between two hydrogen atoms
2) Double covalent bond:- Double covalent bond is formed by sharing of two electron pairs between two atoms in which each atom contributes two electrons. It is represented by putting two short (=) lines between two atoms. e.g.
Formation of oxygen (O₂) molecule:
Oxygen atom has six electrons in its outer most shell. It needs two electrons to complete its octet and attain configuration of neon. Hence, two oxygen atoms combine by sharing two pairs of electrons between them.
3) Triple covalent bond:- Triple covalent bond is formed by sharing of three electron pairs between two atoms in which each atom contributes three electrons. It is represented by three ( ☰ ) short lines between two atoms, e.g.
Formation of nitrogen (N₂) molecule:
Nitrogen atom has five electrons in its outer most shell. It needs three electrons to complete its octet and attain the configuration of the inert gas neon. Hence, two nitrogen atoms combine by sharing of three pairs of electrons between them and form N₂ having triple covalent bond between two nitrogen atoms.
Functional Group: an atom or group of Atoms which largely determines the properties of a compound is known functional group g Alcohols contain hydroxyl (OH) group is functional group
Q Define Homologous series. Give its characteristics.
Ans. A Homologous series may be defined as a family of organic compounds having similar functional group and same chemical properties. Homologous series of alkanes is given below.
Methane Molecular formula
Methane. CH4
Ethane. C₂H6
Propane. C3H8
Butane. C4H10
Pentane. C5H12
Hexane. C6H14
Characteristics of homologous series.
1) All the members of Homologous series can be represented by a general formula e.g. alkanes by Cn H₂n+2
2. Any two adjacent members of a homologous series differ from each other by one carbon and two hydrogen atoms i.e. CH₂ group, by a mass number of 14 units.
3. All the members of a homologous series show similar chemical properties.
4. All the members of a homologous series have the same functional group.
5. The members of a homologous series show a gradation in physical properties.
Q. Discuss the nomenclature of various classes of organic compounds.
Ans:- IUPAC system of Nomenclature:- According to IUPAC system, the name of an organic compound consists of three parts.
1. Word root 2.Suffix 3. Prefix
1. Word root denotes the number of carbon atoms present in the principal chain, which is the longest chain of carbon atoms.
Where C₁,C₂,C3,.......root ........... represents no. of carbon atoms in the chain.
Note:- Extra 'a' given the parenthesis is used only if the primary suffix to be added to the word root start with a consonant.
2. Suffix (a) Primary Suffix... indicates type of bonds. If carbon atoms are linked with single bonds, the primary suffix is 'ane'.
If by double bond the primary suffix is 'ene'
If in triple bond the Primary suffix is 'yne'
(a) Secondary suffix is used to represent the functional group and is attached to primary suffix while writing its IUPAC Name.
(Note:- While adding a secondary suffix to the primary suffix the terminal 'e' of the primary suffix. ane, ene, yne, is replaced by secondary suffix)
(iii) Prefix:- Certain characteristics group are not considered functional groups, these are regarded as substituents such as -F, -CI, -Br, -I etc. Writing the IUPAC name of an aliphatic compound.
Q. Discuss the nomenclature of different classes of organic compounds.
Ans:- The nomenclature of different classes of organic compounds is discussed below:-
1. Alkanes:- General formula CnH2n+2 where n 1.2.3......suffix = ane, names word root = Alk, suffix = ane = alkane
2.Alkenes:- General formula: Cn H₂n Where n = 2,3,4 Functional group: C = C (carbon- carbon double bond))
Suffix: ene
Names:- Replace the terminal "ane" of the corresponding alkane by suffix "ene". The position of double bond is indicated by lowest possible integer. e.g.
n. Formula. Name
2. CH₂=CH₂ Ethene
3. CH3-CH=CH₂ 1 propene
4. CH-CH=CH ーCH3. 2 butene
4. CH3-CH=CH=CH2. 1 butene
4. CH3-CH ーCH2=CH2. 1 butene
3.Alkynes:-
General formula = CnH₂n - 2. Where n = 2, 3, 4,
Functional group: - C ☰ C-(carbon - carbon triple bond.)
Suffix: yne.
Name: Replace the terminal "ane" of the corresponding alkane by suffix "yne" Indicate the position of triple bond by lowest possible integer eg
n. Formula. Name
2. H-C≡ CH. Ethyne
3. HC☰C-CH3. Propyne
4. CH≡C – CH2 – CH3. 1.butyne
4. CH3-C☰C-CH3. 2. butyne
4. CH3 - CH2-CH☰CH. 1 butyne
4.Halo alkanes or alkyl halides.
General formula = RX, when R=Cn H2n+ 1 with n = 1,2,3..... & X = F, Cl, Br, I)
Functional group: F, Cl, Br, I.
Prefix: Floro, chloro, Bromo, lodo for F, Cl, Br, I respectively.
Name: - Add the prefix halo (Fluro, Chloro Bromo, Iodo,) to the parent alkane. Also indicate the position of functional group (F, Cl Br, I) by lowest integer. e.g.
n. Formula. Name
1 (X=CI). CH3 -CI. Chloromethane
2 (X=CI). CH3-CH2-Cl chloro ethane
3 (X=F). CH3-CH2-CH2-F. 1.Floro Propane
3 (X=Br). Br
I
CH3-CH-CH3. 2 Bromo propane
4 (X=I. I-CH2-CH2-CH2-CH3 1 iodobutane
4 (X=F). CH3-CH-CH2-CH3. 2 Fluoro butane
|
F
5.Alcohols:-
General formula: R-OH (Where R=C₂H₂+1 & n = 1,2,3.....…...)
Carbon & its compounds Cha
Functional group: OH (Hydroxyl)
Suffix: ol
Name : Replace last 'e' of parent alkane by "ol'
n. Formula. Name
1. CH3-OH. Methnol
2. CH3-CH2-OH. Ethanol
3- CH3-CH2-CH2-OH. I propanol
3. OH.
|
CH3-CH-CH3 2 Propanol
4. CH3-CH2-CH2-CH2OH. I butanol
4. OH
|
CH3-CH2-CH-CH3. 2 butanol
6.Aldehydes:-
General formula: RCHO
(where R: CnH₂n+land n = 0,1,2,3,4.......)
O
။
Functional group ; ーC一H. (Aldehyde)
Suffix : al
Name: Replace last' e ' of parent alkane by' al'
7.Ketones:-
R' = Cn' H₂n' +1 and n = 1,2,3, and
n' = 1,2,3, also n & n' may be same or different.
O
∥
Functional group :一C一 (Ketone)
Suffix = one
Name = Replace last 'e' of 'ane' by 'one'
e.g.
n. n' Formula
O.
||
CH³ -C- CH3 2-Propanone
8. Carboxylic acids:
General formula: RCOOH where R=CnH₂n+ 1, Where n = 0,1,2
O
||
Functional group: -C- OH (carboxyl)
Suffix oic acid
Name = Replace last 'e' of alkane by 'oic acid'
n Formula. Name
O
॥
0 H-C-OH. Methanoic acid
0
॥
1 CH3-C-OH. Ethanoic acid
0
॥
2 CH3-CH₂-C-OH. Propanoic acid
O
॥
3 CH3-CH₂ - CH₂ - C - OH. Butanic acid
Q .Discuss some important chemical properties of carbon compounds.
Ans:- Some of the important chemical properties of carbon compounds (Hydrocarbon) are discussed as follows:-
1 Combustion:-The process of burning of a carbon compound in air to give carbon dioxide, water, heat and light is known as combustion. Most of the carbon compounds burn in air to produce a lot of heat. e.g. alkanes burn in air to produce a lot of heat, hence are excellent fuels.
When methane burns in sufficient supply of air then carbon dioxide and water vapours are formed with the evolution of large amount of heat.
Combustion
CH⁴ + 2O₂ -------->Co2+ 2H₂O + heat + Light
Saturated hydrocarbons (alkanes) generally burn in air with blue (non- sooty flame.) This is because the percentage of carbon in alkanes is comparatively low- which gets completely oxidized.
Unsaturated hydrocarbons (alkenes and alkynes) burn in air with a yellow sooty flame. This is because the percentage of carbon in alkenes and alkynes is comparatively high.
2 Oxidation Reaction:-Addition of oxygen to any substance is called as oxidation and the substance which is capable of adding oxygen to other substances is called as oxidizing agent. Thus, the reaction in which oxygen is added to any substance is known as oxidation reaction.e.g.
When alcohol is treated with acidified potassium dichromate or alkaline potassium permanganate, it gets oxidized to carboxylic acids.
alkaline. O
KMnO4 ॥
CH3 - CH₂-OH + 2 [0]---------> CH3 C-OH + H₂O
ethanol. acidified. Ethanoic acid
K²Cr2O7
3.Addition reactions.
Due to presence of double and triple bonds, unsaturated hydrocarbons are more reactive and hence add hydrogen in presence of a catalyst such as nickel, platinum or palladium to form saturated hydrocarbons. This process is called as catalytic hydrogenation.
4.Substitution reaction
Reactions which involve the direct replacement (displacement) of an atom or a group of atoms in an organic molecule by another atom or group of atoms without any change in the rest of the molecule are called as substitution reactions. Substitution reaction is an important property of saturated hydrocarbons ( alkanes) e.g. saturated Hydrocarbons in presence of heat and light react with chlorine to form substitution products. e.g.
Sun light
CH4 +Cl₂--------------> CH³. + HCI
Methane chlorine 520-670k. Chloro methane
CH3 Cl + Cl2-----------> CH₂ Cl₂ + Hcl
Dichloromethane
Q.Give a detailed account of ethanol (C₂H5OH).
Ans:-Ethanol is the second member of the homologous series of alcohols. The chemical formula of Ethanol is C₂H5OH. The common name of ethanol is ethyl alcohol and is most common and most widely used.
Physical properties of alcohol.
Some of the physical properties of ethanol are described below.
1) Ethanol is a colourless liquid at room temp. its freezing point is 156K while its boiling point is 351K.
2). It has a distinct smell and a burning taste.
3). Ethanol is soluble in water.
4). It is lighter than water.
5). It has no effect on litmus paper.
Chemical properties of ethanol.
(1)Reaction with sodium:-
Ethyl alcohol is weakly acidic in nature. It evolves hydrogen with active metals like sodium.
2C₂H5OH + 2Na----------->2C₂H5 ONa + H₂
Sodium ethoxide
(2)Combustion reaction:-
It burns in air to form CO₂ and water vapour.
C₂H5 OH + 302-------------->2CO₂ +3H₂O + Energy
(3) Oxidation reaction:-
Ethanol when heated with alkaline potassium permanganate solution or acidified potassium dichromate solution, it gets oxidized to ethanoic acid.
CH3-CH₂-OH+2 [O] Alkaline KMnO→ CH3 COOH + H₂O
or acidified
K₂ cr₂ 07
(4)Dehydration reaction:-
Ethanol when heated with concentrated sulphuric acid at 170°C, it gets dehydrated to form ethane.
Conc. H₂ SO4
CH3-CH₂-OH------------->CH2=CH2 + H2O
Ethanol 170 oC. Ethane
(Dehydration
5 .Reaction with ethanoic acid:-
Ethanol reacts with ethanoic acid on warming in presence of H₂SO4 to form ester.
CH3 COOH + C₂H5OH-------->CH3 COOC2Hs+
Ethanoic acid Ethanol Ester
The reaction in which a carboxylic acid combines with an alcohol to form an ester is called as esterfication reaction. In this reaction
concentrated H₂SO4 acts as dehydrating agent.
Uses:-
(1)Ethanol is used in the manufacture of paints, varmishes, medicines, perfumes, dyes, soaps
and synthetic rubber.
(2)Ethyle alcohol is used in alcoholic drinks like whiskey, beer etc.
(3)It is used as an antiseptic to sterilize the wounds.
(4)Ethyl alcohol is used as a fuel in cars and spirit lamps.
(5)It is used in medicines such as tincture iodine, cough syrups, and many tonics.
(6)Ethanol is used as a solvent for many organic compounds.
Q)What are harmful effects of drinking alcohol?
Ans (1) Alcohol drinking leads to increased road accidents as it effects the nervous system and the person loses the judgment.
(2)Alcohol drinking leads to quarrels and fights which increases the violence and crime in society. This is because alcohol drinking lowers the mental restrain.
(3)Alcohol drinking leads to staggered movement, unclear speech, blurred vision, dizziness and vomiting.
(4)Alcohol drinking makes a person financially bankrupt.
(5)Alcohol drinking for longer periods damages the stomach, liver, heart, brain and ultimately causes death
(6)Adulterated alcohol drinking causes severe poisoning leading to blindness and even death.
Q.Write a short note on denatured alcohol.
A lot of alcohol is used in industries for manufacturing various products. This alcohol is much cheaper than the alcohol available in markets, as it is supplied to the industries tax free. To avoid its miss use, some poisonous substances such as methyle alcohol etc are added to it which make it unfit for drinking purposes. Such alcohol which has been made unfit for drinking is called denatured alcohol.
Continue.......





















0 Comments