NOTES PREPARED BY
ASHAQ HUSSAIN BHAT
TEACHER SCHOOL
EDUCATION DEPARTMENT
JAMMU AND KASHMIR
6:- PHYSICAL AND CHEMICAL CHANGES
points to Remember
- Change is the nature of life.
- Many changes occur in nature daily.
- Changes may be classified as Physical change and Chemical change.
- There is always a cause for a change.
- cannot be controlled.
- Some changes can be controlled while some other changes
- Physical change is a temporary change and can be reversed.
- In a physical change, no new substance is formed.
- A chemical change is a permanent change and cannot be easily reversed.
- In a chemical change, new substances with entirely different properties are formed.
- Changes can be grouped together by finding similarities between them.
- Properties such as shape, size, colour and state are called physical properties
- . A change in which a substance undergoes a change in its physical properties is called physical change.
- Ribbon of magnesium burns with a brilliant white Iight
- When carbon dioxide is passed through lime wate., lime water turns milky.
- Chemical changes may accompany with sound, light, heat, smell, gas, colour change.
- . Burning is a chemical change and is always accompanied by production of heat.
- Ozone acts as a protective layer in the atmosphere.)
- Rusting needs the presence of both oxygen and water.
- Iron is protected by depositing a layer of zinc on it and this process of depositing zinc on other metal is called galvanization
- Stainless steel is a mixture of carbon, chromium, nickel and manganese.
- Iron can also be painted to avoid rusting.
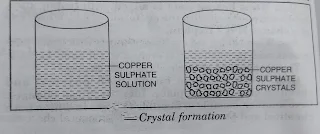
- Crystallization is the process of obtaining large particles of definite geometrical shape of a compound from its saturated solution.
IMPORTANT DEFINITIONS
Physical change. The change in which physical properties of substance change and no new substance with new properties are formed is called physical change, e.g. powdering a lump of sugar.
Chemical change. The change due to which the substance changes completely to form new substances having new properties is called chemical change, e.g. burning of paper.
Rusting. The process of acquiring brownish film by iron when kept in moist air is called rusting.
Galvanization. The process of depositing a layer of zinc on iron to prevent it from rusting is called galvanization.
Crystallization. The process of obtaining large crystals of a substance from its solution is called crystallization.
TEXT BOOK EXERCISES
Q. 1. Classify the changes involved in the following process as physical or chemical change :
(a) Photosynthesis
(6) Dissolving sugar in water,
(c) Burning of coal
(d) Melting of wax.
(e) Beating aluminium to make aluminium foil.
(f) Digestion of food.
Ans.(a) Photosynthesis-Chemical change.
(6) Dissolving sugar in water-Physical change
(c) Burning of coal-Chemical change
(d) Melting of wax-Physical change
(e) Beating aluminium to make aluminium foil—Physical change
(f) Digestion of food-Chemical change.
Q. 2. State whether the following statements are true or false. In case a statement is false, write the corrected statement in your notebook.
(a) Cutting a log of wood into pieces is a chemical change. (True/false)
(b) Formation of manure from leaves is a physical change. (True/false)
(c) Iron pieces coated with zinc do not get rusted easily. (True/false)
(d) Iron and rust are the same substances. (True/false)
(e) Condensation of steam is not a chemical change. (True/false)
Ans.(a) False--cutting a log of wood into pieces is a physical change.
(6) False--formation of manure from leaves is a chemical change.
(c) True--Iron pieces coated with zinc do not get rusted easily.
(d) False-Iron and rust are not same. Rust is iron oxide.
(e) True-Condensation of steam is not a chemical change.
Q. 3. Fill in the blanks in the following statements
(a) When carbondioxide is passed through lime water, it turns milky due to formation of.................
(b) The chemical name of baking soda is.................
(c) Two methods by which rusting of iron can be prevented are.................. and................
(d) Changes in which only................... properties of a substance change are called physical changes.
Ans. (a) Calcium carbonate (b) Sodium bi-carbonate
(c) Painting, galvanization (d) physical.
Q. 4. When baking soda is mix with lemon juice, bubbles are formed with the evolution of gas. What type of a change is it? Explain.
Ans. When baking soda (a base) is mixed with lemon juice (a weak acid) carbondioxide is evolved which forms bubbles. This change is an example of chemical change.












1 Comments
Very simple and precise notes good going dear sir
ReplyDelete