NOTES PREPARED BY
ASHAQ HUSSAIN BHAT
TEACHER SCHOOL
EDUCATION DEPARTMENT
JAMMU AND KASHMIR
"STRUCTURE OF THE ATOM"
Introduction
The smallest particle of an element that can exist chemically is an atom. Dalton assumed that the atom is indivisible. But experiments in late 1800s and early 1900s revealed that the atom is made up of three subatomic particles - electrons, protons and neutrons. The atom is neutral in nature since it has equal number of negatively and positively charged particles.
Electrical Nature of Matter
The first important and experimental evidence of electrical nature of matter was established by Faraday in 1833. He showed that the flow of electricity is due to the flow of charged particles. The term electron was first suggested by G.J. Stoney for unit charge on a monovalent negative ion. Existence of electrons was later proved by J.J. Thomson
Charged Particles in Matter
Cathode Rays - Discovery of Electron
William Crookes, a British scientist, noted that gases are ordinarily poor conductors of electricity. However, when a high voltage (10,000 volts) charge from an induction coil is applied to discharge tube filled with gases at very low pressure (0.01 mm of mercury), the gases become good conductors of electricity and electricity begins to flow in the form of rays. These rays are called cathode rays, since these rays originated from the negative plate i.e., cathode and travel from the cathode towards the anode. Later J.J. Thomson studied the characteristics and constituents of cathode rays in detail
which led to the discovery of electron in 1897.
A common discharge tube is a long glass tube having two metal plates sealed at its two ends. These metal plates are known as electrodes. The electrode which is connected to the positive terminal of the battery is known as anode (positive electrode), and the electrode which is connected to the negative terminal of the battery is called cathode (negative electrode).
When air inside the discharge tube is at the atmospheric pressure and a high electric voltage of 10,000 volts (or more) is applied to the electrodes, no electricity flows through the air in the discharge tube
If the pressure of air inside the discharge tube is reduced by pumping out the gas with the help of
a vacuum pump to about 1 mm of mercury and high voltage is applied again, electricity begins to
flow through air and a light is emitted by the air inside the tube. The colour of light changes with the nature of gas taken in the discharge tube.
When the pressure of air in the discharge tube is reduced to about 0.001 mm of mercury and a high voltage is applied to the electrodes, the emission of light by air stops. Though inside of the discharge tube now appears to be dark, the walls of the discharge tube at the end opposite to the cathode begin to glow with a greenish light called fluorescence. This shows that some invisible rays are formed at the cathode and when these rays strike the glass tube, they emit a greenish light. Since these rays are formed at the cathode, they are known as cathode rays.
Properties of Cathode Rays
- Cathode rays travel in straight lines: If a solid object is placed in the path of cathode rays a sharp shadow of the object is formed on the wall opposite to the cathode. It shows that cathode rays travel in straight lines
- Cathode rays consist of material particles: This was indicated by the fact that a light paddle wheel placed in the path of cathode rays starts rotating.
- Cathode rays carry negative charge : When an electric field is applied to a stream of cathode rays, they get deflected towards positive plate of the electric field. It shows that cathode rays are negatively charged.
- Cathode rays produce heating effect: Cathode rays heat the object on which they fall due to transfer of kinetic energy to the object.
- Effect of magnetic field: When magnetic field is applied perpendicular to the path of cathode rays, they get deflected in the direction expected for negative particles. This further confirmed that cathode rays are negatively charged.
- On striking against walls of the discharge tube, cathode rays produce faint greenish fluorescence.
- Cathode rays ionize the gas through which they pass.
- Cathode rays produce X-rays when they are made to fall on metals such as tungsten, copper, etc.
- They can penetrate through thin metal foil.
- The charge to mass ratio (e/m) for the particles in the cathode is independent of the nature of the gas taken in the discharge tube or the nature of the cathode.
- It was concluded that cathode rays produced from different gases are same and negatively charged particles present in them are also same. These particles were given the name electrons which was proposed by Johnstone Stoney.
Characteristics of an Electron
- Charge on an electron: The absolute charge on an electron is-1.602x 10-¹9coulombs or-4.8×10-¹0esu. It was determined by Millikan oil drop experiment . This quantity of charge has been shown to be the smallest negative charge carried by any particle and is usually referred to as unit negative charge.
- Mass of an electron : The mass of an electron is about 1/1837 the mass of a hydrogen atom. Actual mass of an electron is 9.11 × 10-28g and is negligibly small.
- Charge to mass ratio: The charge to mass ratio (e/m) for an electron (cathode rays particle) was found to be 1.76 × 10¹¹ C/kg or 1.76 ×108 C/g.
- Electron is represented by the symbol_10e.
- Thus, an electron may be defined as a sub-atomic particle which carries one unit negative charge (1.622 ×10-19C) and has a mass (9.1 × 10-³¹kg) equal to 1/1837th of that of hydrogen atom.
Discovery of Proton - Study of Anode Rays or Canal Rays
The formation of cathode rays has shown that all the atoms contain negatively charged particles called electrons. Now, an atom is electrically neutral, so it must contain some positively charged particles to balance the negative charge of electrons. It has actually been found by experiments that all the atoms contain positively charged particles called protons. The existence of protons in the atom was shown by Goldstein.
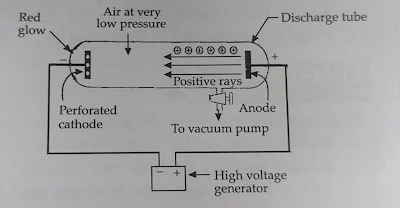
In the production of positive rays a discharge tube having perforated cathode is used. A perforated cathode is a cathode having holes in it. These perforations or holes allow the positive rays to pass through them
When a high voltage of about 10,000 volts is applied to a discharge tube having a perforated cathode .and containing air at very low pressure of about 0.001 mm of mercury, a faint red glow is observed behind the cathode
These rays are formed at the anode and when these rays strike the walls of the discharge tube they produce a faint red light. Since these rays are formed at the anode (positive electrode), they are known as anode rays or positive rays.
Anode rays are also known as canal rays because they pass through canals of the cathode.
In a discharge tube, cathode rays are emitted from the cathode. These rays consist of stream of
electrons which move towards anode with very high speed. When these electrons strike the atoms or molecules of the gas in the discharge tube, one or more electrons are knocked off from the atoms or molecules of the gas and results in the formation of positively charged ions. These positive charged ions (particles) of the gas constitute the anode rays.
Properties of Anode Rays
- Anode rays travel in straight lines.
- Anode rays consist of material particles.
- Anode rays are deflected by electric field towards negatively charged plate. This indicates that these rays are positively charged.
- When a magnetic field is applied in a direction perpendicular to the path of anode rays, they get defected in the direction expected for positive particles. This further indicates that these rays are positively charged
- Charge to mase ratio of the particles in the anode rays depends upon nature of the gas taken in the discharge tube
- When hydrogen gas is taken in the discharge tube the positive particles (H) produced in the anode rays are called protons
Characteristics of a Proton
Mass of a proton: The mass of a proton is 1837 times that of an electron. The relative mass of a
proton is equal to 1.005757 amu which is taken to be equal to 1 amu. The absolute mass of a proton
is 1.672 ×10-²⁴
Charge on a proton: The charge on a proton is equal in magnitude but opposite in sign to that of an electron The charge carried by a proton is equal to 1.602× 10-¹9C which is taken to be one unit of positive charge
(ie+1). Thus, a proton is said to carry a unit positive charge.
A proton is represented by the symbol 1¹p.
Discovery of Neutron
Until 1920, an atom was supposed to consist of only two fundamental particles, ie, protons and
electrons. Since electrons has negligible mass, the center of mass of the atom was regarded as the mass of proton only. Rutherford found that except for hydrogen atom, the atomic mass of no other elements could be explained by electrons and protons only. For example, the element Li ion has 3 protons in the nucleus of its atom. Therefore, the mass of the lithium atom must be thrice the mass of the proton. But, its mass was actually six times the mass of proton. To solve this problem, Rutherford predicted the presence of another type of particle which must be electrically neutral and has a mass almost equal to that of a proton
- In 1932, James Chadwick bombarded the element beryllium with 𝜶-particles.
9 4 12 1
Be + He ---------->C+ n 4 2 6 0
He observed the emission of radiations with the following properties :
- The radiation was highly penetrating.
- The radiation remained unaffected in an electric or magnetic field, i.e. the radiation was neutral.
- The particles constituting the radiation had the same mass as that of the proton. Thus, the relative Mass of such a particle - 1 amu and the absolute mass = 1.6× 10-²⁴ g. Because of their electrical neutrality, these particles were called neutrons.
- Mass of a neutron : The absolute mass of a neutron is 1.6 × 10-²⁴ g. The mass of a neutron is equal to the mass of a proton hence its relative mass is 1 u.
- Charge of a neutron : Neutron has no charge. It is electrically neutral.
- A neutron is represented by the symbol 10n
Atomic Models of an Atom
Thomson's Model of an Atom
Limitation :
Rutherford's Model of an Atom
Rutherford's 𝜶-particle scattering experiment - Discovery of nucleu
In 1911, Rutherford performed 𝜶-particles scattering experiment which led to the downfall of Thomson's model. The experiment involved the bombardment of a thin sheet of heavy metallike gold (thickness - 100 nm or 10cm) by 𝜶 -particles. Alpha particles are positively charged helium nuclei. carries a mass of 4 u and a charge of +2 units, c-Particles were obtained from radioactive substance like radium placed in the cavity of a block of lead and made into a fine beam with a slit. A circular fluorescent screen coated with zine sulphide (Zn) was placed around the fol to detect the deflection suffered by c-particles. Whenever an e-particle struck the screen, a tiny flash of light was produced at that point.
- Most of the o-particles (nearly 99%) passed through the gold foil undeflected.
- Some of the 𝜶-particles were deflected by small angles.
- A very few ex-particles (1 in 12,000) were either deflected by very large angles or were actually reflected back along their path.
Rutherford explained his observation as follows:
most of the c-particles passed through the foil undeflected, it indicates that the most of the space in an atom is empty -Particles being positively charged and having considerable mass, could be deflected only by some heavy, positively charged centre. The small angle of deflection of a particles indicated the presence of a heavy positive centre in the atom. Rutherford named this positive centre as nucleus. 𝜶-Particles which make head-on collision with heavy positive centre are deflected through large
Main Features of Rutherford's Nuclear Model
- An atom consists of two parts: nucleus and extra nuclear portion.
- Nucleus is present in the centre of the atom and is surrounded by extra nuclear portion.
- The size of the nucleus is very small as compared to that of the atom.
- Almost the entire mass of an atom is concentrated in the nucleus. All the protons and neutrons (discovered later on by Chadwick) are present in the nucleus. The particles present in the nucleus i.e., protons and neutrons are collectively called nucleons.
- All the electrons are present in the extra nuclear space around the nucleus.
- Electrons present in the extra nuclear portion are not stationary. These are revolving around thenucleus at high speed following a circular path.
- Thus, Rutherford's model is comparable to solar system in which the electrons revolve around the nucleus as the planets revolve around the Sun.














0 Comments